|
 |
| J Korean Soc Geriatr Neurosurg > Volume 19(2); 2023 > Article |
|
Abstract
Objective:
Bone morphogenetic protein 2 (BMP-2) shows promise in clinical applications for promoting bone fusion. However, high doses of BMP2 can lead to adverse effects. As an alternative, the activin A/BMP-2 chimera (AB204) has been studied for its potential to overcome the limitations of BMP2 and enhance bone formation. In this clinical trial, we intended to evaluate the efficacy of AB204 for bone fusion efficacy and stability in patients requiring one-level spinal fusion.
Methods
We conducted a single-blind, randomized, active-controlled clinical trial to evaluate the efficacy and stability of AB204 for bone fusion. Interbody fusion was performed by inserting AB204 or a bone autograft into the cage of patients requiring one-level transforaminal lumbar interbody fusion operation. Patients in the AB204 group received calcium phosphate with AB204 (2 mg), whereas those in the control group received only local bone transplants. At 24 weeks after surgery, computed tomography and radiography were performed to assess the degree of interbody fusion. The visual analog scale, Oswestry disability index, and perioperative data were also analyzed.
Results
Through the direct administration of AB204, successful bone fusion was achieved in all patients in the AB204 group, with no side effects such as ectopic bone formation, vertebral osteolysis, cancer, or inflammation. In the control group, one patient exhibited Bridwell grade II fusion along with screw loosening, whereas the remaining patients experienced no complications.
With the progression of aging, the number of spine surgeries performed in elderly patients has increased, along with an increase in the incidence of osteoporosis among spinal surgery patients [1]. Osteoporosis is characterized by decreased bone density and quality, leading to an increased risk of fractures. Consequently, patients with osteoporosis often experience a higher incidence of failure in bridging bone formation after spinal fusion surgery [2,3]. Recent research has shown promising results in the clinical application of bone morphogenetic protein 2 (BMP-2) to enhance bone fusion rates, leading to active exploration of its clinical applications [4-6]. However, the use of high doses of BMP-2 to induce sufficient bone fusion may lead to adverse effects, such as ectopic bone formation, vertebral osteolysis and/or edema, neuritis, cancer, and inflammation [7-9].
To overcome the limitation of BMP-2, several new bone substitutes have been introduced, among which the activin A/BMP-2 chimera (AB204) is currently being researched as a potential alternative to BMP-2. AB204 showed resistance to the BMP-2 antagonist noggin, and enhanced bone formation through a synergy effect [10,11]. Recent rat model experiments conducted at our institution confirmed that AB204 exhibits superior bone fusion properties compared to BMP-2 [12,13]. These findings imply that lower concentrations of AB204 may yield similar bone-fusion effects while reducing the side effects associated with BMP-2 administration. This clinical trial aimed to evaluate the efficacy and stability of AB204 in patients who underwent one-level spinal fusion.
This clinical trial was designed as a single-blind, randomized, and active control study to investigate the efficacy of AB204 in one-level transforaminal lumbar interbody fusion (TLIF). The inclusion criteria were male and female patients aged 20 years or above but below 80 years who required one-level spinal fusion due to conditions such as spinal stenosis, spinal instability, herniated disc, spondylolisthesis, or retrolisthesis. Exclusion criteria included patients diagnosed with osteoporosis with a T score of less than -2.5 as a result of dual-energy X-ray absorptiometry; those who had previously undergone spinal fusion surgery; those with recent fractures at the surgical site; those who underwent tumor removal at the surgical site or were diagnosed with malignant tumors; those with infections, pregnant or lactating women; and those who exhibited hypersensitivity to AB204.
All the patients provided detailed information regarding the course of the study. All study procedures were approved by the Institutional Review Board (IRB) of Inha University Hospital (IRB No. 2018-06-026).
All surgeries were performed using one-level TLIF. The patients underwent general anesthesia and were placed carefully in the prone position. The surgical procedure was performed using the posterior open midline approach. Pedicle screw placement was performed using the freehand technique and X-ray was performed to confirm screw positioning. For cage insertion, total laminectomy and unilateral facetectomy at the dominant pain side was performed to ensure protection of the neural structures, and the intervertebral disc was excised using pituitary forceps. The cartilaginous end plates were decorticated using shavers and curettes. In the AB204 group, the interbody fusion cage was filled with calcium phosphate and 2 mL AB204 (Biobetter Biologics Inc., Incheon, Korea) and inserted into the interbody space. In the control group, local bone harvested from decompression sites, such as facets, laminae, and processes, was used to fill the cage and was subsequently inserted. For completing the fixation of the vertebrae, pedicle screws are attached to rods. After confirming that the neural structures are sufficiently decompressed, drains are inserted, and the wound is closed with dissolving sutures.
For clinical evaluation, the visual analog scale (VAS) score was analyzed preoperatively and at 1 and 24 weeks postoperatively. The Oswestry disability index (ODI) was also evaluated before and 24 weeks postoperatively. In addition, the operative time, estimated volume of intraoperative blood loss, hospitalization period after the operation, and time until ambulation after the operation were analyzed. At 24 weeks post-surgery, all participants underwent radiography and computed tomography (CT) scans to evaluate the status of bone fusion. The interbody fusion rate was analyzed using the anterior fusion grade described by Bridwell et al. (Table 1) [14].
Eight patients were enrolled in this study, 4 assigned to the AB204 group and 4 to the control group. Patient demographics and perioperative data are summarized in Table 2. Regarding underlying diseases, the AB204 group comprised 3 cases of degenerative disc disease and one case of degenerative spondylolisthesis, whereas the control group had one case of degenerative disc disease and 3 cases of degenerative spondylolisthesis. Analyzing surgical anesthesia records, the mean operation time was 140±10.80 and 190±51.15 minutes, with average intraoperative blood loss volumes of 225±95.74 and 350± 173.21 mL in the AB204 and control groups, respectively. Considering hospitalization records, the average postoperative hospitalization periods were 11.25±2.50 and 11.75±3.50 days, and the average time to postsurgical ambulation was 3±1.41 and 2±1.15 days in the AB204 and control groups, respectively.
In terms of clinical evaluation outcomes, the VAS scores decreased from an average of 6.5±1.29 points preoperatively to 2.25±0.50 points after 24 weeks of surgery in the AB204 group, and from 6.25±0.95 points to 3±0.81 points in the control group. As for ODI, the scores decreased from 35.75±9.17 preoperatively to 16.25±8.01 postoperatively in the AB204 group, and from 34.5±16.66 to 17±12.98 in the control group (Tables 3, 4).
At 24 weeks after TLIF, all patients in the AB204 group showed Bridwell fusion grade I on CT and no definite instability on flexion-extension X-ray examination. Fig. 1 depict the CT and X-ray images taken 24 weeks after surgery of patients in the AB204 group.
In the control group, 3 of 4 patients showed Bridwell fusion grade I, while one patient demonstrated Bridwell fusion grade II. A patient with Bridwell fusion grade II also showed evidence of screw loosening on CT. However, no definite instability was observed on flexion-extension radiographs, and the patient did not complain of any distinct clinical symptoms (Fig. 2). No significant complications including neurological deterioration or heterotopic ossification were observed in either group.
Autografts continue to be the gold standard for spinal fusion and are regarded as the optimal bone graft owing to their comprehensive support of bone fusion through osteogenesis, osteoinduction, and osteoconduction. Its safety profile is remarkable as it eliminates the risk of infection transmission associated with grafting. The overall complication rate increases with additional pain and morbidity at the donor site, prolonged operation time, and anesthesia time. Since traditional open lumbar fusion uses autologous bone chips obtained during nerve decompression, including intraoperative laminectomy, these problems have been offset and widely used. However, as surgical approaches such as anterior lumbar interbody fusion (ALIF) and lateral lumbar interbody fusion or procedures such as minimally invasive lumbar interbody fusion or endoscopic lumbar interbody fusion gain popularity, procedures that either do not involve bony element harvesting or have limitations in autograft extraction are becoming increasingly important.
According to a recent meta-analysis, the fusion rate after 24 months in a group using BMP-2 in posterolateral fusion surgery was 94% [15]. Another long-term follow-up study showed a high fusion success rate of 98% when BMP-2 was inserted during ALIF surgery [4]. BMP-2 is widely used as a bone graft substitute in spinal fusion surgery. However, the use of high doses of BMP-2 to induce proper fusion has been associated with side effects such as ectopic bone formation, bone cyst formation, inflammation, and cancer formation [9]. Therefore, there is a demand for new alternatives, and various studies on new materials have been conducted.
In this study, the use of AB204 as a bone substitute for lumbar fusion resulted in consistently favorable initial clinical outcomes. Notably, clinical indicators such as VAS and ODI demonstrated successful reductions in both groups, regardless of the type of bone substitute employed. However, the AB204 group exhibited robust fusion outcomes, whereas the control group, which used autografts, experienced asymptomatic screw loosening. When TLIF was performed by inserting AB204, no complications were observed that could occur when BMP-2 was used.
Transforming growth factor (TGF)-β family proteins play a crucial role in various cellular processes, including development, homeostasis, and physiology. They are involved in controlling cell survival or apoptosis, extracellular matrix deposition, and specific cell proliferation, particularly during osteoblast differentiation and bone formation [16]. The TGF-β family encompasses over 30 members in humans, including BMP, activins, and growth differentiation factors [17].
BMPs predominantly form complexes with ALK1, ALK2, ALK3, or ALK6 and activate the SMAD1/5/8 pathway. Activins, in contrast, activate the SMAD2/3 pathway through the type I activin receptors ALK4 and ALK7 [18,19]. Despite these differences, both BMPs and activins bind to type II receptors ActRII and ActRIIb, and activins exhibit a higher affinity for type II receptors than BMP-2 [20,21]. In a previous study, chimeric ligand AB204 was created by replacing the type II receptor epitope of BMP-2 with activin A. In vitro and in vivo experiments have demonstrated that AB204 exhibits superior osteogenic and bone healing activities compared to BMP-2 [10]. In our institution, experiments conducted on normal and osteoporotic rats showed that AB204 displayed similar or better bone fusion properties than rhBMP-2 at equivalent concentrations [12,13].
This study had several limitations. First, the study’s pool was small, which hindered a statistically robust analysis of the clinical outcomes within the control group. Moreover, the relatively brief 24-week follow-up period limited the comprehensive evaluation of the clinical results. Additionally, as this study did not entail a direct head-to-head comparison between AB204 and BMP-2, it was not feasible to ascertain the superiority of either treatment. Another noteworthy point is that the outcomes were analyzed exclusively from the perspective of a single surgeon, thereby inhibiting the generalizability of the findings. Consequently, future investigations should prioritize prospective studies involving a larger participant cohort and an extended follow-up period. With a larger patient cohort and extended follow-up period, there may be cases of nonunion with reduced fusion rates or we may identify new complications associated with AB204. This approach enabled a comprehensive comparison between groups treated with AB204 and those undergoing lumbar fusion with autogenous bone or BMP-2. Furthermore, given that BMP-2 leads to considerable soft tissue swelling, it is prudent to expand safety assessments to other areas. Despite these limitations, it is essential to recognize the significance of this study. This significance stems from the direct assessment of AB204's efficacy through its implantation into human individuals.
We confirmed the effectiveness of AB204 in achieving spinal fusion by applying AB204 to patients requiring one-level spinal fusion surgery. Radiologically, the bone fusion rates were good and no other complications were observed. We believe that AB204 can potentially serve as a new bone substitute to overcome the complications associated with BMP-2.
Fig. 1.
Preoperative sagittal T2-weighted image, bone fusion on computed tomography sagittal view and flexion-extension X-ray images taken 24 weeks postoperatively in the AB204 group. (A) 46-years-old women who underwent L5/S1 transforaminal lumbar interbody fusion (TLIF). (B) 59-years-old man who underwent L4/5 TLIF. (C) 48-years-old women who underwent L5/S1 TLIF. (D) 73-years-old women who underwent L5/S1 TLIF. Bone bridge formation on the CT image is indicated by a red arrow.
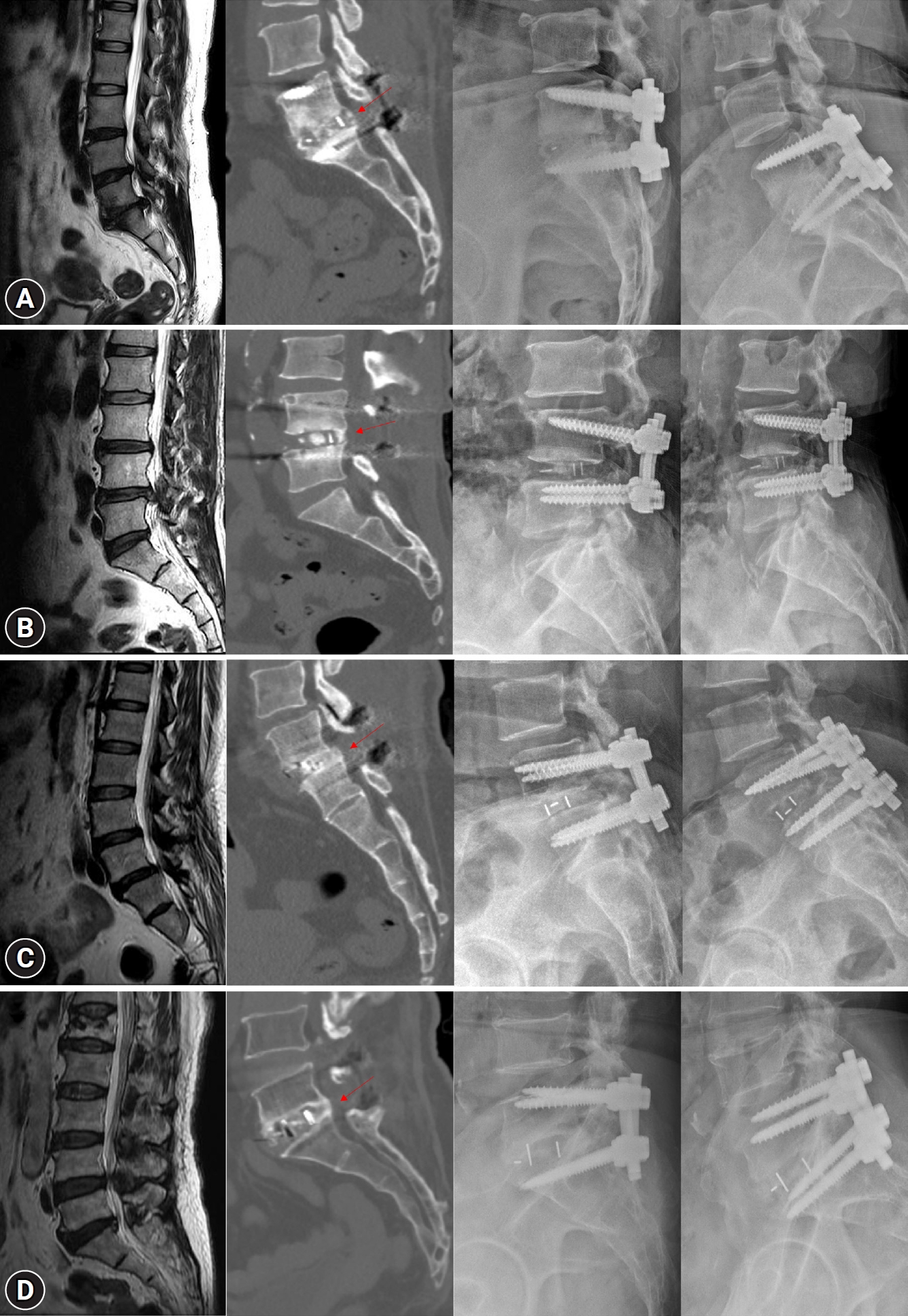
Fig. 2.
Computed tomography and flexion-extension X-ray images of a patient in the control group revealing Bridwell fusion grade 2 and screw loosening (indicated by the red arrow).
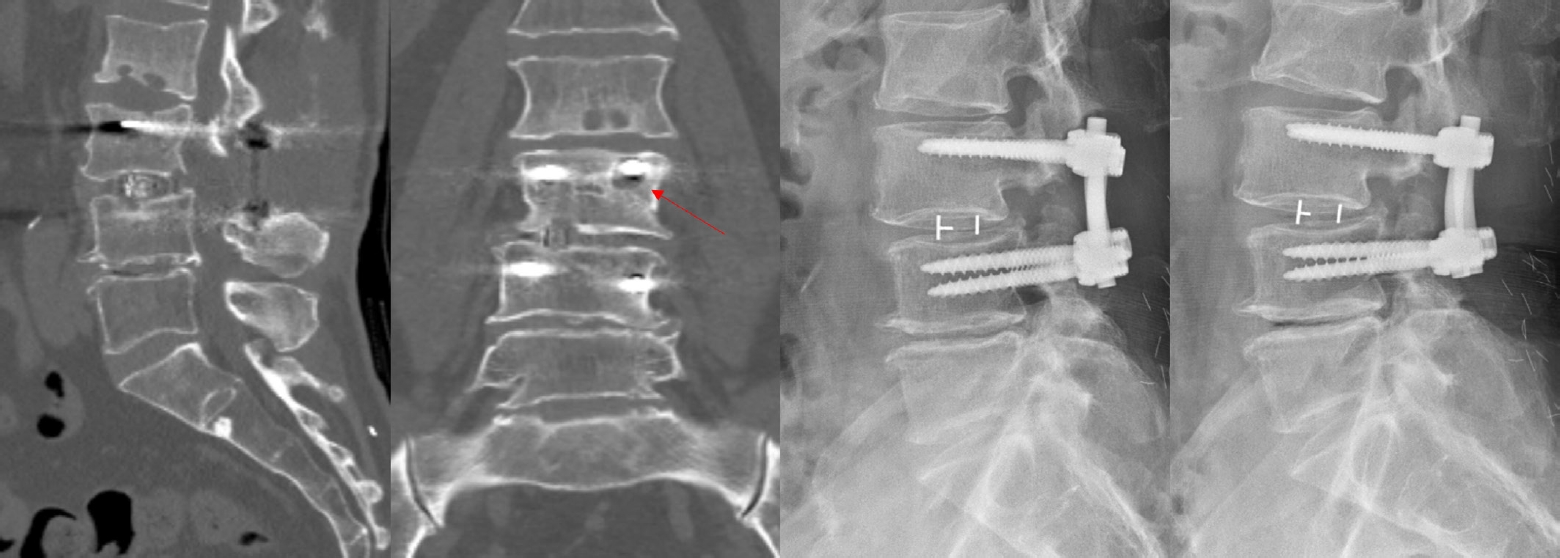
Table 1.
Bridwell fusion grades and computed tomography fusion analysis results after 24 weeks
Table 2.
Demographic and perioperative data of patients
REFERENCES
1. Chin DK, Park JY, Yoon YS, et al. Prevalence of osteoporosis in patients requiring spine surgery: incidence and significance of osteoporosis in spine disease. Osteoporos Int 2007;18:1219-24.


3. Glassman SD, Carreon L, Dimar JR. Outcome of lumbar arthrodesis in patients sixty-five years of age or older. Surgical technique. J Bone Joint Surg Am 2010;92 Suppl 1 Pt 1:77-84.


4. Smoljanovic T, Siric F, Bojanic I. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am 2010;92:2614-5.

5. Muchow RD, Hsu WK, Anderson PA. Histopathologic inflammatory response induced by recombinant bone morphogenetic protein-2 causing radiculopathy after transforaminal lumbar interbody fusion. Spine J 2010;10:e1-6.

6. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J 2008;8:1011-8.


7. Tannoury CA, An HS. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J 2014;14:552-9.


8. Smoljanovic T, Bojanic I, Vlahovic Z. Safety of posterior interbody fusions of the lumbar spine using rhBMP-2. J Spinal Disord Tech 2010;23:78.


9. James AW, LaChaud G, Shen J, et al. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng Part B Rev 2016;22:284-97.



10. Yoon BH, Esquivies L, Ahn C, et al. An activin A/BMP2 chimera, AB204, displays bone-healing properties superior to those of BMP2. J Bone Miner Res 2014;29:1950-9.



11. Zheng GB, Yoon BH, Lee JH. Comparison of the osteogenesis and fusion rates between activin A/BMP-2 chimera (AB204) and rhBMP-2 in a beagle's posterolateral lumbar spine model. Spine J 2017;17:1529-36.


12. Son S, Yoon SH, Kim MH, Yun X. Activin A and BMP chimera (AB204) induced bone fusion in osteoporotic spine using an ovariectomized rat model. Spine J 2020;20:809-20.


13. Ryu D, Yoon BH, Oh CH, et al. Activin A/BMP2 chimera (AB204) exhibits better spinal bone fusion properties than rhBMP2. J Korean Neurosurg Soc 2018;61:669-79.




14. Bridwell KH, Lenke LG, McEnery KW, Baldus C, Blanke K. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine (Phila Pa 1976) 1995;20:1410-8.


15. Mariscal G, Nuñez JH, Barrios C, Domenech-Fernández P. A meta-analysis of bone morphogenetic protein-2 versus iliac crest bone graft for the posterolateral fusion of the lumbar spine. J Bone Miner Metab 2020;38:54-62.



16. Chen G, Deng C, Li YP. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 2012;8:272-88.



17. Morikawa M, Derynck R, Miyazono K. TGF-β and the TGF-β family: context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol 2016;8:a021873.



18. ten Dijke P, Yamashita H, Ichijo H, et al. Characterization of type I receptors for transforming growth factor-beta and activin. Science 1994;264:101-4.


19. Tsuchida K, Nakatani M, Yamakawa N, Hashimoto O, Hasegawa Y, Sugino H. Activin isoforms signal through type I receptor serine/threonine kinase ALK7. Mol Cell Endocrinol 2004;220:59-65.


- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 883 View
- 6 Download
- ORCID iDs
-
Tae Min Cheon

https://orcid.org/0009-0002-7596-9820Dalsung Ryu

https://orcid.org/0000-0003-0895-3431Seung Hwan Yoon

https://orcid.org/0000-0003-0558-2313 - Related articles



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print