Introduction
Intracranial schwannomas account for approximately 5% to 8% of primary intracranial tumors and most commonly originates from the 8th cranial nerve [
1]. In fact, intracranial intraparenchymal schwannoma (IS) that are not derived from CN VIII have rarely been reported. Since the initial report by Gilbson et al. in 1966, only approximately 150 cases have been reported [
2]. This type of schwannoma is slow growing and typically observed in young people under the age of 30; however, symptoms can manifest quickly in old age [
3].
Case Report
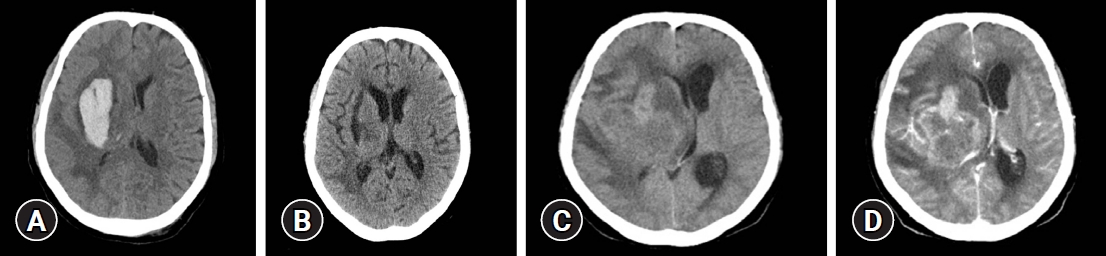
A 65-year-old woman presented to the emergency department with impaired consciousness and underwent an emergency burr hole procedure for management of spontaneous intracerebral hemorrhage (ICH) with intraventricular hemorrhage in the right basal ganglia (BG) (
Fig. 1). The ICH biopsy revealed a hematoma, not specific. The patient received treatment for hypertensive ICH and was transferred to another hospital. Approximately 3 months later, she was brought to another hospital due to decreased consciousness, subsequently, a brain computed tomography (CT) was performed which revealed a right BG mass-like lesion and was transferred to this hospital for further examination. The patient was in stupor at the time of discharge from the first hospitalization; however, she recovered well, apart from minor concerns of drowsiness and left hemiparesis.
A previously performed CT scan showed a 62×45 mm irregular mass with heterogeneous enhancement and approximately 14 mm midline shift (
Fig. 2). In the brain magnetic resonance imaging performed later at our hospital the T1 weighted image (T1WI) showed slight gray matter hypointensity, and the T2 weighted image (T2WI) showed peritumoral edema and heterogeneously hyperintense lesions, while contrast-enhanced T1WI revealed intense ring enhancement of the mass. Based on these findings, the lesion was suspected to be glioblastoma multiforme (GBM).
Therefore, the patient underwent a craniotomy and tumor removal. Following right frontotemporoparietal craniotomy and dural incision, the mixed friable and hard, grayish, poorly demarcated, marginated mass (4.5×5×4 cm) was accessed using the transcortical approach (
Fig. 3). It was then removed using a cavitron ultrasonic surgical aspirator since there was no involvement of the ependymal lining of the ventricles. Further, no sign of necrosis along with vascular proliferation was observed by analysis of the frozen biopsy sample, leading to a suspicion of glioma.
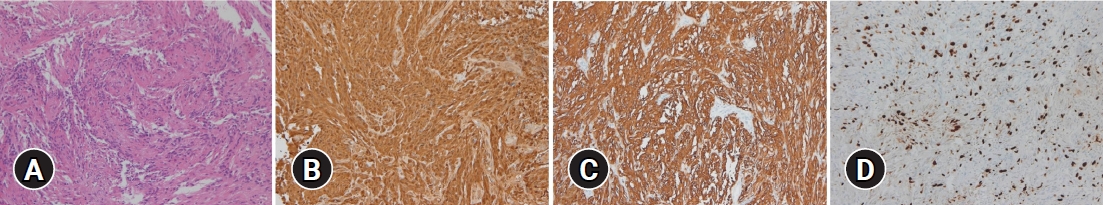
Upon histopathological examination, the cellular lesions consisted of a bundle of spindle cells arranged in a storiform pattern with palisading arrangement; however, no nuclear pleomorphism was seen. In addition, mild infiltration was of the mass into the brain parenchyma surrounding the tumor cells was observed, indicating the presence of a neurogenic tumor due to ovoid to spindle and partially wavy or buckled shaped cells. Immunohistochemical staining showed strong expression of both S-100 and glial fibrillary acidic protein (GFAP), while Ki-67 labeling index was 39.9% and the mitotic count was 3-4/10 high-power filed (
Fig. 4).
Discussion
IS is an extremely rare intracranial tumor. Despite being prevalent in various age groups, from the youngest case being of a patient aged 6 months to the oldest case one being 84 year-old, it frequently occurs in individuals under 30 years of age (58.7%). Of these, 12.0% (18) of patients were over 60 years of age, which is unexpected. The incidence was higher in men in the age group under 30 years (male:female; 1.84:1); whereas, the pattern was reversed in the group above 60 years of age (male: female; 0.46:1) (
Table 1) [
4-
8].
IS may originate anywhere in the intracranial region; however, appears to be more frequent in the supratentorial region during young age, whereas it is commonly located in the infratentorial region in individuals >60 years of age [
4]. Although the pathophysiology of IS has not yet been clearly identified, 2 hypotheses have been proposed: (1) developmental theory, (2) non-developmental theory. The developmental theory assumes that mesenchymal pial cells in the brain parenchyma transform into Schwann cells. Alternatively, the non-developmental theory assumes that Schwann cells originate from adjacent organs, such as the meningeal branch of the perivascular nerve plexus and anterior ethmoidal and trigeminal nerves, where Schwann cells usually exist [
9]. At present, it unclear clear which of these 2 hypotheses prevails.
The radiologic features of IS include cyst formation, calcification, and peritumoral edema, and it is difficult to find distinct differences from those of vestibular schwannomas [
5,
10]. However, only one similar case with intratumoral hemorrhage has reported so far, and even in vestibular schwannoma, the features shown in this case is known to be very rare, accounting for about 0.4% [
6,
11].
The diagnosis of intraparenchymal schwannoma by imaging is challenging, and is dependent on histopathological analysis and immunohistochemical findings. The histopathological appearance was characterized by Antoni A and B cells, while immunohistochemical staining showed positive expression of S-100 whereas epithelial membrane antigen and GFAP were absent in most cases [
4,
7].
Since intraparenchymal schwannoma presents with slow proliferation, Ki-67 labeling index is usually observed at less than 1% [
1]. In this case, high Ki-67 labeling index was observed, indicating rapid growth, similar to a malignant intracerebral nerve sheath tumors (MINST). MINST is termed malignant peripheral nerve sheath tumors (MPNST) of brain parenchyma. MINST’s characteristics is similar to MPNST’s one except not associated of neurofibromatosis type Ⅰ. In histologically, MINST has pleomorphic cells with irregular nuclei. And that tumor also showed poorly differentiated malignant spindle cell. MINST is extremely rare, only one case was reported in the age group above 60 years [
8]. The malignancy rate was approximately 5% in the age group above 60 years, which was slightly lower compared to other age groups [
4].
IS are mostly benign tumors and show a good prognosis after total resection [
1]. It is crucial to set an accurate surgical goal in the early stages of IS treatment for better clinical outcomes; however, there are certain limitations in the diagnosis of IS since it is difficult to differentiate from GBM or high-grade gliomas based on the imaging findings. Thus, hematoxylin and eosin staining is the only feasible option for examination of intraoperative frozen biopsy [
7].
Conclusion
IS is a benign lesion characterized by slow growth in most cases and presents a good prognosis when treated with total resection. To the best of our knowledge, this is the first case of IS characterized by a rapid growth rate without evidence of malignancy. Since it is difficult to rule out GBM in the early stages, gross total resection and short-term follow-up will be required for management of these tumors.













 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print