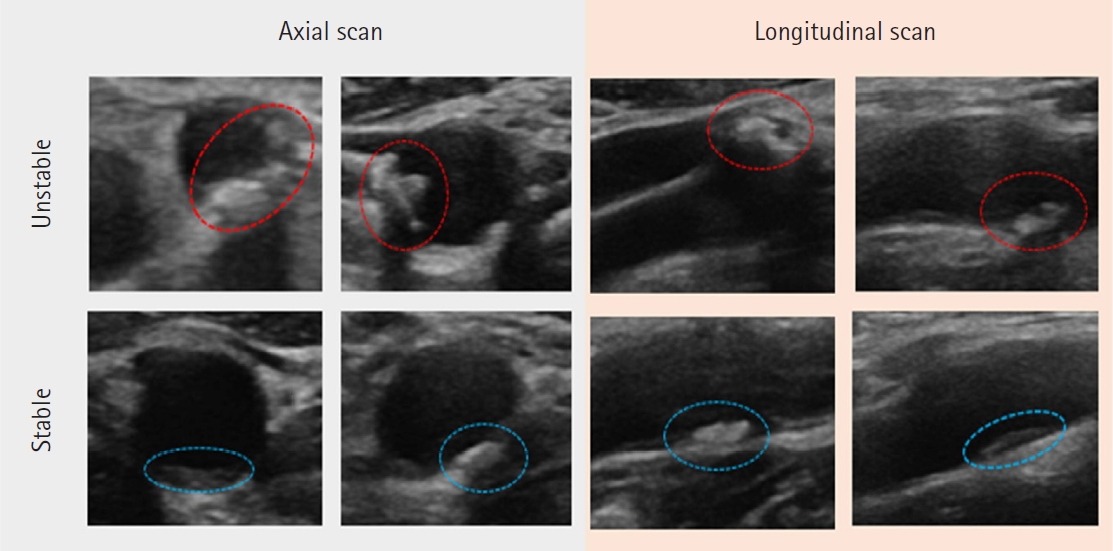
Carotid plaques form various shapes and occur in areas from the distal common carotid artery to proximal internal carotid artery [
13]. It is more accurate to predict patient prognosis and choose treatment methods based on plaque vulnerability rather than only the degree of carotid stenosis. The major features used to describe carotid plaques in carotid US images include calcification, ulceration, hemorrhage, and a lipid-rich necrotic core, as well as IMT [
10]. Calcification with ulceration is stimulated by prolonged shear stress and sudden arterial pressure changes on the carotid bifurcation [
14]. This activates inflammation and metalloproteinase activity, causing erosion of the calcification to progress to ulceration of the surface [
15]. Ulceration in the carotid plaque predisposes to thrombus formation. Plasminogen activator inhibitor-1 within plaques creates a thrombotic effect [
16]. The expression of tissue factors, which have a major effect on the coagulation cascade, is determined by the extent and degree of inhibition of the tissue factors pathway within the plaque [
17]. Tissue factor is localized to areas of lipid deposition and cholesterol crystals in carotid plaque [
18]. Bos et al. [
19] reported that intraplaque hemorrhage of the carotid artery significantly increased the risk of stroke (hazard ratio, 2.42; 95% CI, 1.30-4.50). Considering these facts, the accurate classification of carotid plaque vulnerability (unstable vs. stable plaque) is important for clinicians to determine treatment plans. Three radiologic tests, MRI, CT, and US are available to examine plaque vulnerability in the carotid artery. MRI provides excellent differentiation of soft tissue composition with high resolution, but its use can be limited due to the high medical costs and time-consuming procedures needed to obtain the images [4-6]. CT has the advantage of shorter imaging time than MRI but has the limitation that a contrast agent is required to distinguish plaque components [
7,
8]. In the domestic medical environment, MRI and CT is used in more than US in small and medium-sized general hospitals. And in most of these hospitals, radiologists are available to interpret plaque vulnerability. Compared to MRI and CT, carotid US is widely used in private hospitals, as well as for health examinations in general and university hospitals in Korea due to its relatively low cost, ease of using at the bedside, and non-invasiveness. Lukanova et al. [
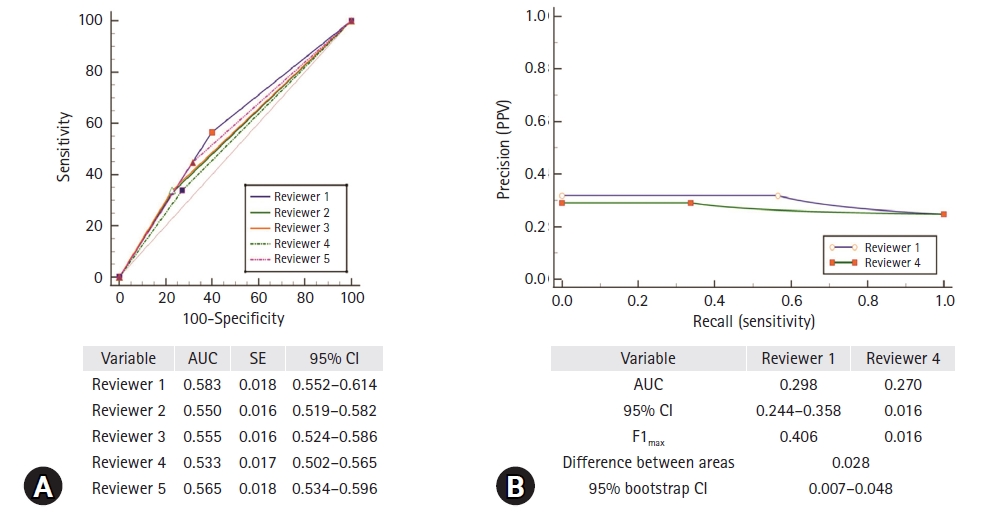
20] reported that carotid US showed high balanced accuracy for detecting unstable plaques with a sensitivity of 94% and specificity of 93% compared to multi-detector CT, with a sensitivity of 83% and specificity of 73%, and MRI with a sensitivity of 100% and specificity of 89%. However, the procedure and its interpretation may vary depending upon the examiner’s experience. In particular, considering the reality of private hospitals with many non-radiologists, it is necessary to properly classify plaque vulnerability. In this study, we evaluated the accuracy of plaque vulnerability classification, targeting neurosurgical residents. Although there were some differences in classification accuracy between the reviewers, the overall accuracy level was low (0.5<AUC≤0.7) [
21]. Considering that stroke risk is largely dependent upon not only the degree of stenosis but also plaque vulnerability, accordingly, education on discriminating plaque vulnerability is necessary during resident training [
22]. However, it is not easy to educate residents on carotid US interpretation given the reality of medical situations. In this case, a new computer-aided diagnostic system using machine learning can be an alternative. Tanaka et al. [
23] demonstrated that a convolutional neural network (CNN) composed of VGG19 and ResNet152 showed accurate classifications of breast ultrasound images with a sensitivity of 90.9% (95% CI, 84.5%-97.3%), specificity of 87.0% (95% CI, 79.5%-94.5%), and AUC of 0.951 (95% CI, 0.916-0.987). Biswas et al. [
24] proposed a two-stage artificial intelligence model to measure atherosclerotic wall thickness and total plaque burden. However, they did not distinguish plaque vulnerability. Lekadir et al. [
25] also suggested a CNN for automatically characterizing plaque components, such as the lipid core, fibrous cap, and calcified tissue. However, they used only 56 cases. Accordingly, there is a need for artificial intelligence (AI) based on gray-scale carotid US images, which is often used in the field, and AI research that not only detects plaque composition but comprehensively informs on plaque vulnerability.
There were some limitations in this study. First, there were only five participants, and they were from the same hospital. Thus, a concern may arise as to whether the residents participating in the study actually represent non-radiologists working in private hospitals. Second, the correct answer was decided only by one neurointerventionist. Although he had more than 20 years of experience, selection bias may have occurred, and this should be taken into account when interpreting our results. Nevertheless, to the best of our knowledge, this was the first study to investigate the classification accuracy of plaque vulnerability by neurosurgical residents based on carotid US images in actual clinical settings.













 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print